1. Engineering the Unbreakable: The Shift from Borosilicate Glass to COP/COC
In my 20 years of engineering experience within the biopharmaceutical packaging sector, I have never seen a shift as critical as the one happening right now. For decades, Type I Borosilicate Glass was the undisputed king of vaccine vials. But the cracks in the kingdom are showing—literally. Glass delamination, breakage during lyophilization (freeze-drying), and the catastrophic risk of breakage during ultra-cold chain transport (-80°C) have forced the industry to look for alternatives.
Enter Cyclo-Olefin Polymer (COP) and Cyclo-Olefin Copolymer (COC). These materials offer the transparency of glass but with the durability of plastic and a superior moisture barrier. However, molding COP/COC is an art form. These materials are expensive and thermally sensitive. Traditional two-step blow molding processes introduce too much thermal stress and particulate risk.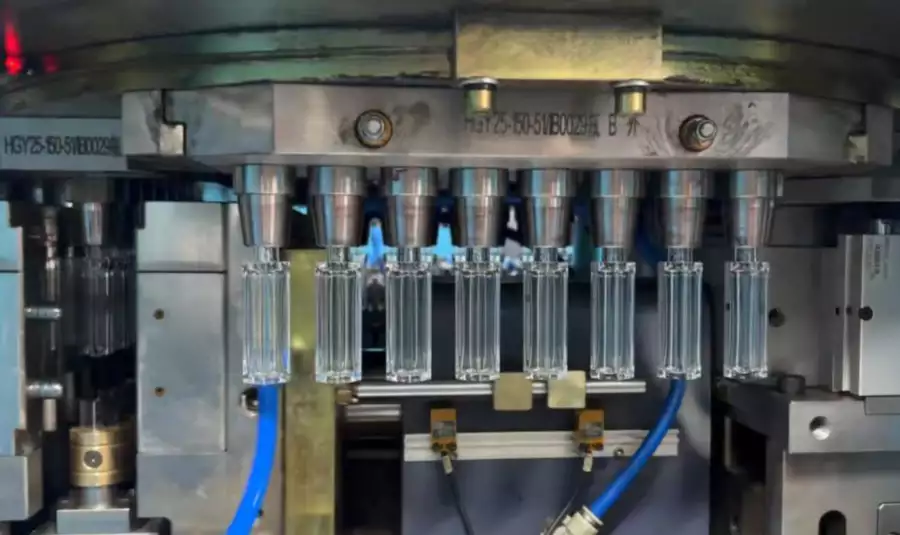
This is where the One Step Blow Molding Machine (ISBM) technology becomes the only viable path forward. By integrating injection and blowing into a single, sterile, temperature-controlled cycle, we can produce vials that meet the most stringent USP <661.1> standards without the risks of glass.
2. Why Vaccine & Biological Vials Demand One-Step ISBM
For biologicals, the container is not just a package; it’s part of the drug delivery system. The interaction between the vial surface and the protein-based drug is critical.
- Particulate-Free Production: One-Step ISBM is a “melt-to-solid” process within a closed loop. There is no trimming, no regrind, and no human handling of preforms, eliminating the risk of particulate contamination which is fatal for injectable drugs.
- Precise Neck Dimensions: Vaccine vials require crimp seals (aluminum caps). The dimensional tolerance of the lip is critical for seal integrity. ISBM injection molds the neck finish, guaranteeing a perfect ISO-standard crimp surface every time.
- Optical Clarity: COP/COC processed via our ISBM-maskin retains glass-like clarity, allowing for automated visual inspection of the vaccine liquid.
3. Core Biopharma Needs vs. Ever-power Technology Matching
| Biopharma Pain Point | Ever-power ISBM Solution | Technical Outcome |
|---|---|---|
| Delamination & Breakage Glass failure at low temps. |
COP/COC Processing Capability Specialized Screw & Barrel Design. |
Vials withstand -196°C (Liquid Nitrogen) without cracking or delaminating. |
| Protein Adsorption Drug sticking to glass walls. |
Inert Surface Finish High-polish mold cavities. |
COP surface is hydrophobic and inert, minimizing drug loss and protein aggregation. |
| Sterility Assurance Contamination during molding. |
Full Electric Cleanroom Design Servo-driven, no hydraulic oil. |
ISO Class 7 compatible. Zero risk of hydraulic oil mist contamination. |
| Dimensional Stability Crimp seal failure. |
Injection Molded Neck High-pressure formation. |
Neck dimensions within ±0.05mm, ensuring 100% Container Closure Integrity (CCI). |
4. Typical Sub-Segment Applications
Our One Step Injection Blow Molding technology is deployed for high-value biological containers:
COVID-19 & mRNA Vaccine Vials
2ml, 6ml, and 10ml vials made of COP. These require extreme cold resistance and must replace glass seamlessly on filling lines.
Lyophilization (Freeze-Dry) Vials
Thick-bottomed vials designed to withstand the stress of freeze-drying cycles without the breakage rates seen in glass.
High-Viscosity Biologics
Silicone-free pre-fillable syringe barrels and larger vials for monoclonal antibodies, where glass interaction is a risk.

5. Ever-power Solution: The HGY150-V4-EV Advantage
For COP/COC vials, standard PET machines will fail. The material is expensive (up to $20/kg) and behaves differently thermally. We recommend the HGY150-V4-EV (4-Station, Full Servo).
Why this configuration?
- 4-Station Precision: The dedicated Conditioning Station is non-negotiable for COP. It allows us to precisely temper the preform temperature to the narrow processing window of cyclo-olefins, ensuring optical clarity and preventing “pearlescence” (stress whitening).
- Full Servo Control (EV): COP has high melt viscosity. Our servo-driven injection unit provides the powerful yet precise pressure needed to fill the mold cavity without inducing shear stress, which causes haze.
- Scrap Reduction: With COP costing 15x more than PET, our “zero-scrap” hot runner system and high-precision servo controls ensure every gram of material becomes a sellable vial.
6. Process Flow: The 4-Station Sterile Cycle
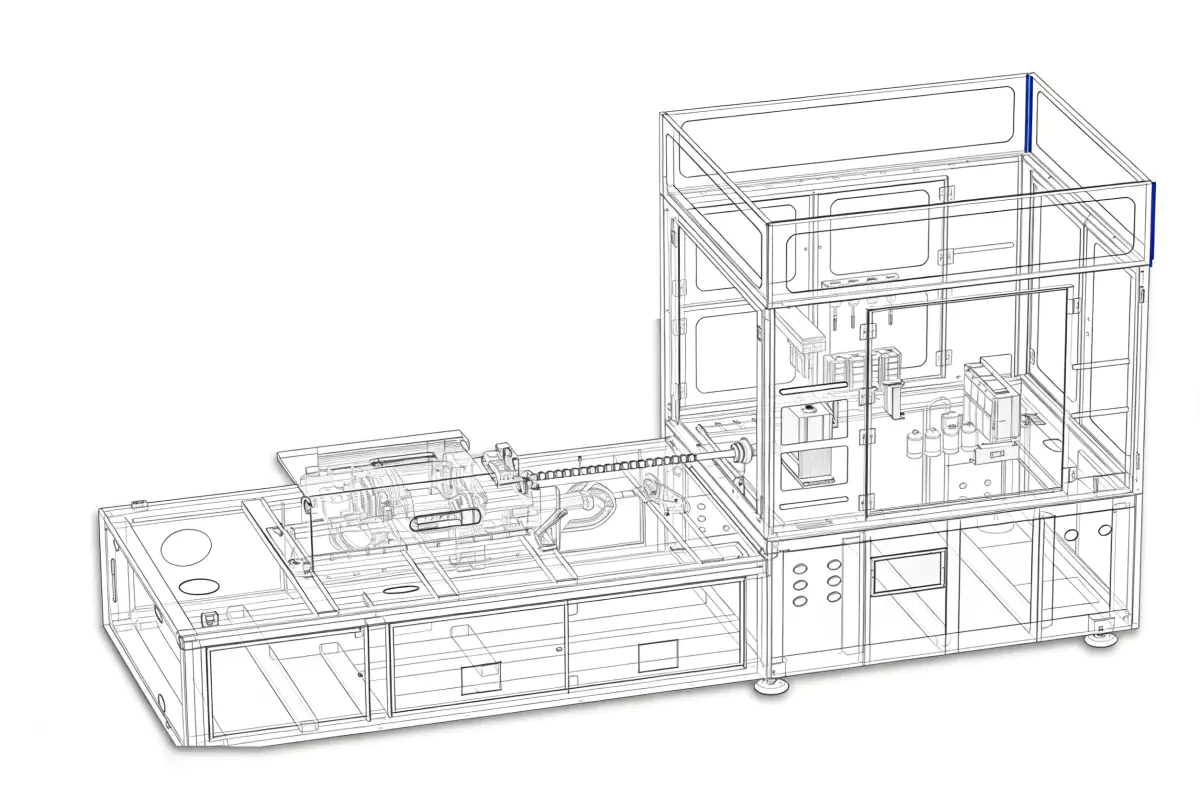
Our One Step PET Bottle Machine (adapted for COP) follows a rigorous 4-step sterile journey:
Molten COP is injected into the cavity. The crimp neck is formed.
Thermal homogenization. Critical for preventing optical defects.
Clean air expands the vial. Biaxial orientation increases strength.
Robot removal to cleanroom conveyor. No scratches.
7. Engineer’s Field Notes: Global Success Cases
[Switzerland] High-Value Oncology DrugChallenge: Glass vials were delaminating, causing particulate recalls for a $5,000/dose drug.
Solution: Transitioned to COP vials using HGY150-V4-EV.
Result: Zero delamination. FDA approval granted for plastic primary container.
[USA] mRNA Vaccine Supply ChainChallenge: Needed storage vials capable of -80°C transport without breakage.
Solution: One Step Injection Molding Machine optimized for COC.
Result: Passed all drop tests at cryogenic temperatures. Breakage rate reduced from 3% (glass) to 0%.
[South Korea] CMO for BiologicsChallenge: High cost of imported Japanese COP vials.
Solution: In-house production with Ever-power ISBM technology.
Result: Reduced unit cost by 50%. ROI achieved in 14 months despite high machine and mold costs.
[China] Animal Health VaccinesChallenge: Massive volume requirement (100 million units) with strict sterility.
Solution: Fleet of HGYS280-V6 machines running continuously.
Result: Became the market leader in unbreakable vaccine packaging.
8. Value Analysis: In-House COP Vial Production
Purchasing ready-made COP vials is incredibly expensive (approx. $0.50 – $1.00 per unit). Making them in-house changes the economics drastically:

- Cost Per Unit: Raw COP material cost per vial is a fraction of the purchased finished good price. Savings can exceed 60%.
- Supply Chain Security: Dependence on a few global glass suppliers is risky. In-house ISBM production secures your supply.
- Zero Breakage Loss: Eliminating product loss due to container breakage during filling and lyophilization saves millions in lost drug product.
10. The Verdict: One-Step ISBM vs. Glass/Other Plastics
| Feature | Ever-power One-Step ISBM (COP) | Borosilicate Glass | Two-Step Plastic Molding |
|---|---|---|---|
| Breakage Risk | Zero (Unbreakable) | High (Fragile) | Low |
| Particulates | None (Melt formed) | Glass flakes/Delamination | Dust from preform storage |
| Dimensional Precision | High (Injection Molded) | Medium (Tube forming) | Medium (Reheat variation) |
| Cryogenic Storage | Excellent (-196°C) | Risk of cracking | Material dependent |
11. Regulatory Compliance: Meeting Global Pharma Standards
Transitioning from glass to plastic requires rigorous validation. Ever-power machines facilitate this:
- USP <661.1> & <661.2>: Our process ensures COP vials meet all physicochemical tests for plastic packaging systems.
- Extractables & Leachables (E&L): The pure, additive-free processing in our machines minimizes potential leachables profile.
- ISO 15378: Our machine manufacturing follows GMP principles suitable for primary packaging materials for medicinal products.
12. Brand Comparison: Ever-power vs. Market Leaders
While companies like Nissei ASB pioneered this field, Ever-power offers a compelling value proposition for biologics manufacturers:
| COP Expertise | Specialized screw designs for Daikyo Crystal Zenith® and Topas® COC processing. |
| Capital Expenditure | 40% Lower Investment allows for rapid scaling of vaccine production lines. |
| Turnkey Support | We provide mold design, machine validation (IQ/OQ), and material sourcing guidance. |
*Disclaimer: Trademarks belong to their respective owners. Comparison for technical reference only.
13. FAQ: Expert Answers for Biopharma Engineers
Is COP difficult to process?
It is more sensitive than PET. It requires precise temperature control to avoid haze. Our V4 machines with conditioning stations are specifically tuned for this.
Can I sterilize these vials?
Yes. COP/COC vials produced on our machines are compatible with Autoclave (steam), Gamma irradiation, and E-Beam sterilization.
What sizes can I make?
We can mold vials from 2ml up to 100ml on the same machine platform by changing molds.
Do you offer cleanroom shrouding?
Yes, we offer stainless steel HEPA filter enclosures (LAF units) mounted directly on the clamping unit to maintain ISO 5 conditions at the mold open phase.
How do you handle the high cost of COP material?
Our hot runner systems are designed for zero waste. Sprues are minimal or eliminated (valve gate), ensuring maximum material yield.
Can I run PET on the same machine?
Yes. The machine is versatile. By changing screw/barrel parameters and molds, you can switch between COP for high-value drugs and PET for standard applications.
What is the cycle time for a 10ml vial?
For a COP vial, typical cycle times are around 12-14 seconds on a 4-station machine, prioritizing optical quality over raw speed.
14. Critical Companion: Oil-Free Air Compressors
For sterile ISBM Blow Molding Machine operations, the blowing air is a critical utility. It must be sterile and oil-free.
We supply integrated ISO 8573-1 Class 0 Oil-Free Air Compressors to ensure that the air expanding your vaccine vial is as pure as the vaccine itself.

Secure Your Biological Supply Chain
Transition to unbreakable, sterile COP/COC vials with Ever-power ISBM technology.

