1. Engineering Sterile Precision: The Shift to One-Step ISBM in Pharma
In my 20 years of engineering experience within the medical packaging sector, I have witnessed a critical transformation. The days of accepting basic extrusion blow molding for premium ophthalmic solutions are fading. Why? Because the modern pharmaceutical industry demands tighter tolerances, higher sterility assurance levels (SAL), and absolute freedom from particulates.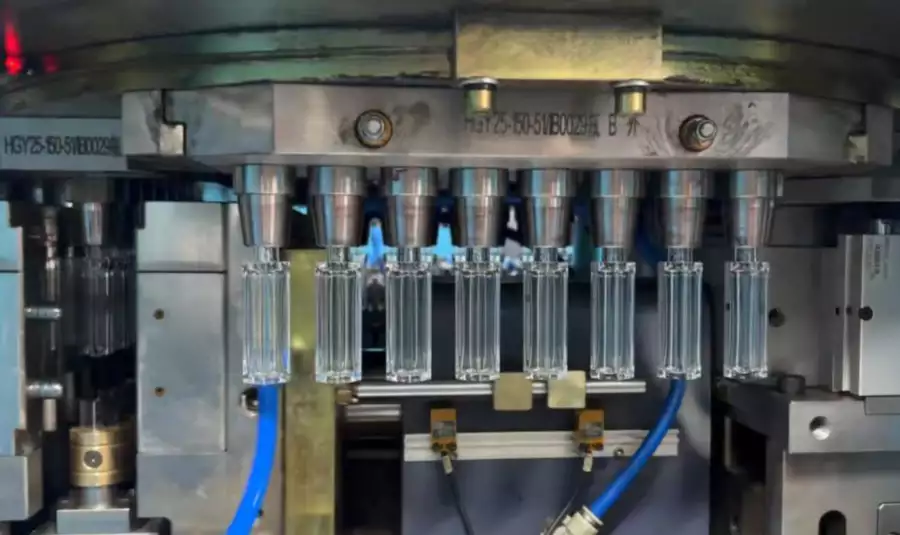
For eye drop bottles (Ophthalmic Bottles), specifically in the 5ml to 15ml range, the challenges are microscopic but monumental. Traditional two-step processes or extrusion methods often struggle with neck finish precision. A leaking eye drop bottle is not just a defective product; it is a sterility breach that can recall an entire batch. Furthermore, the flash generation in extrusion creates particulate risks that are unacceptable in cleanroom environments.
This is where the One Step Blow Molding Machine (ISBM) technology changes the game. By integrating the injection of the preform and the blowing of the bottle into a single, enclosed, and heat-retained cycle, we eliminate human intervention and environmental exposure. For pharmaceutical giants, this isn’t just machinery; it’s a compliance strategy.
2. Why Ophthalmic Packaging Requires One-Step ISBM
The anatomy of an eye drop bottle is deceptive. It looks simple, but the interaction between the bottle neck, the dropper insert (nozzle), and the tamper-evident cap is a feat of engineering. The tolerance allowed is often less than ±0.05mm.
- Injection Molded Neck: Unlike extrusion, ISBM injects the neck. This guarantees that the thread start, the locking ring for the cap, and the inner diameter for the plug are dimensionally perfect every shot. No reaming or trimming required.
- No Regrind/Flash: One-step ISBM is a zero-waste process. There is no flash to trim, which means no dust particles floating in your ISO 7 cleanroom.
- Material Versatility: While LDPE is common for squeezability, modern preservative-free formulations often use special PP or PET grades for better barrier properties. Our ISBM Bottle Machine handles these with ease.
3. Core Pharma Needs vs. Ever-power Technology Matching
| Pharmaceutical Pain Point | Ever-power ISBM Solution | Technical Outcome |
|---|---|---|
| Contamination Risk Hydraulic oil leaks in cleanrooms. |
Full Electric (EV) Series Servo-driven, oil-free molding area. |
Meets GMP & ISO Class 7/8 requirements. Zero oil contamination. |
| Dosage Consistency Inconsistent squeeze force. |
Micro-Injection Control Precision screw position control. |
Wall thickness deviation <0.03mm, ensuring consistent drop size. |
| Seal Integrity Leaking caps during transport. |
Direct Injection Neck High-pressure molded threads. |
Perfect mating with dropper plugs and caps. |
| Small Batch Efficiency High changeover costs for small vials. |
Compact 3-Station Design HGY50-V3 structure. |
Faster cycles for small bottles (5-15ml) and quicker mold changes. |
4. Typical Sub-Segment Applications
The ophthalmic market is diverse. Our machines are currently producing:
Standard Eye Drop Bottles
5ml, 10ml, 15ml bottles made of LDPE or PP. The focus is on squeezability and memory (returning to shape).
Preservative-Free Systems (PF)
Complex rigid bottles (often PP or PET) designed to house multi-dose preservative-free pumps (OSD systems). These require extreme neck precision to hold the pump mechanism.
Contact Lens Solution Vials
Travel-sized 10ml-20ml bottles that require high transparency (PET) to show liquid clarity.

5. Ever-power Solution: The HGY50-V3-EV Advantage
For the Ophthalmic sector, we do not recommend a one-size-fits-all machine. We recommend the HGY50-V3-EV (Full Servo). This is the “Ace” for small pharmaceutical containers.
Why the HGY50-V3-EV?
- Full Servo Drive (The “EV” Factor): In a cleanroom, hydraulic oil is the enemy. Our EV series uses servo motors for mold clamping, injection, and stretching. This eliminates the risk of oil leakage contaminating the sterile field, a prerequisite for GMP compliance.
- Micro-Gram Control: Eye drop bottles are tiny. A weight variation of 0.1g affects the “squeeze” feel. Our servo injection unit controls the screw position to the micrometer, ensuring every bottle feels exactly the same in the patient’s hand.
- Compact 3-Station Efficiency: Small bottles cool fast. They don’t need the 4th station conditioning time required by heavy jars. The 3-station design (Inject-Blow-Eject) minimizes the machine footprint in expensive cleanroom real estate and optimizes the cycle time for small shot weights.
6. Process Flow: The 3-Station Sterile Cycle
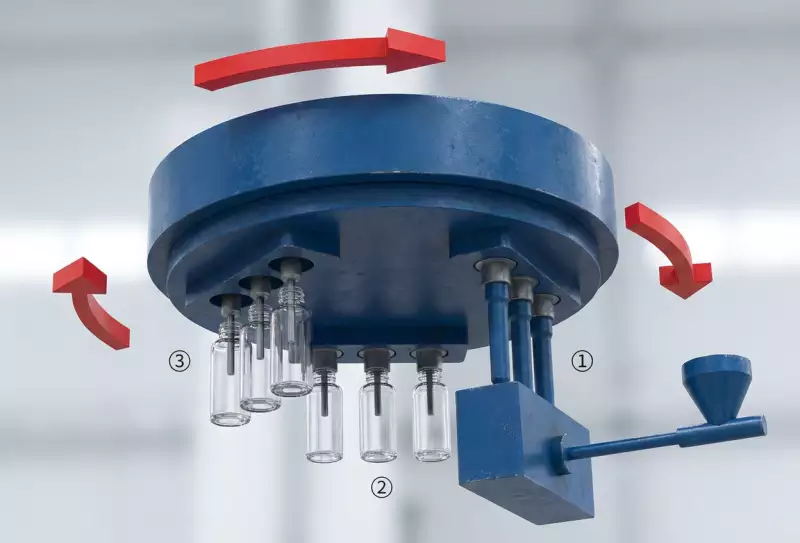
1. Micro-Injection
Resin is melted and injected into a high-precision cavity. The neck finish is fully formed and cooled here.
2. Stretch & Blow
The preform is indexed 120°. A servo rod stretches it axially while clean air blows it radially to shape.
3. Automated Ejection
The finished bottle is stripped and transferred to the conveyor, ready for inline leak testing.
7. Engineer’s Field Notes: Global Success Cases
[Germany] Preservative-Free Pump BottleChallenge: Client needed a rigid PP bottle with extreme neck tolerance (±0.03mm) to fit a patented airless pump. Extrusion blowing failed on leakage tests.
Solution: HGY50-V3-EV with High-Cavitation Mold.
Result: 0% leakage rate. The injection-molded neck provided the perfect seal for the snap-on pump.
[USA] Generic Eye Drop ManufacturerChallenge: Replacing glass dropper bottles with PET to reduce shipping weight and breakage.
Solution: One Step Injection Blow Molding Machine (ISBM) running medical-grade PET.
Result: 80% weight reduction. Passed USP <661> testing for containers.
[India] High-Volume CMOChallenge: Producing 10 million units/month of 5ml LDPE bottles in an ISO 7 cleanroom.
Solution: Multiple HGY50-V3-EV units. The oil-free design reduced their cleanroom filtration load significantly.
Result: Met FDA audit requirements with zero observations regarding machinery hygiene.
[Brazil] Veterinary OphthalmologyChallenge: High cost of imported 2-step bottles.
Solution: In-house production using Ever-power ISBM.
Result: ROI achieved in 9 months due to material savings and logistics elimination.
[Japan] Premium Contact Lens CareChallenge: Surface scratches on bottles were deemed “unacceptable quality” for the Japanese market.
Solution: One-Step process eliminates preform tumbling (scratching).
Result: “Glass-like” cosmetic quality achieved in plastic.
8. Value Analysis: ROI for In-House Production
Moving from purchasing bottles to manufacturing them with Ever-power’s One Step Blow Molding Equipment yields significant returns, typically within 12-18 months.

- Material Cost vs. Bottle Cost: Raw resin is significantly cheaper than buying finished sterile bottles. Savings often exceed 40%.
- Inventory Reduction: Produce on demand. No need to store millions of empty bottles (air) in expensive warehouses.
- Sterility Assurance: By molding and filling in the same facility (or inline), you reduce the bio-burden risk chain.
10. The Verdict: One-Step vs. Two-Step for Eye Drops
| Feature | Ever-power One-Step (ISBM) | Traditional Two-Step / Extrusion |
|---|---|---|
| Neck Precision | Perfect (Injection Molded) | Variable (Calibrated/Trimmed) |
| Hygiene/Cleanliness | High (Sterile Cycle) | Risk (Storage/Trimming Dust) |
| Flash/Scrap | Zero | High (Extrusion tails/moils) |
| Space Requirement | Compact (1 Machine) | Large (Injection + Reheat Blower) |
| Energy Efficiency | High (Retains Heat) | Low (Reheating from cold) |
11. Global Regulatory Compliance & Safety
We understand that in pharma, compliance is not optional. Ever-power machines are built to support your validation process:
- Cleanroom Ready: Compatible with ISO 7 (Class 10,000) and ISO 8 (Class 100,000) environments. Stainless steel shrouding available.
- Material Traceability: All parts contacting resin are SS316L or food-grade certified, supporting FDA 21 CFR compliance.
- DQ/IQ/OQ/PQ Support: We provide full documentation packages to assist your engineering team in validating the machine onsite.
12. Brand Comparison: Ever-power vs. Market Leaders
We respect the Japanese pioneers (ASB, Aoki). However, Ever-power offers a strategic advantage for agile pharma manufacturers:
| Technology | Compatible 3-Station/4-Station Architecture. Proven Servo Design. |
| Cost Efficiency | 30-40% Lower CAPEX allowing for redundancy investments (buying 2 machines for the price of 1 competitor machine to ensure uptime). |
| Lead Time | Rapid deployment (3-4 months) vs. 9-12 months industry average. |
*Disclaimer: Trademarks belong to their respective owners. Comparison for technical reference only.
13. FAQ: Expert Answers for Pharma Buyers
Can I produce sterile bottles directly?
While the molding process involves high heat (sterilizing the plastic melt), the machine environment must be controlled. Our machines are designed to operate under Laminar Air Flow (LAF) hoods to maintain sterility at the ejection point.
What is the smallest bottle I can make?
We routinely produce 3ml and 5ml eye drop bottles. The HGY50-V3-EV is optimized for these small shot sizes.
Can I use Medical Grade PP?
Yes. Polypropylene (PP) is common for squeezable eye drop bottles. Our screws are designed to handle the shear characteristics of medical PP without degradation.
Is the machine oil-free?
Our “EV” series is fully electric in the molding zone, eliminating hydraulic oil leaks. Some auxiliary movements may use enclosed pneumatics, but the risk of oil contamination is removed.
Do you support IQ/OQ validation?
Yes, we provide the necessary technical documentation and onsite support to help your Quality Assurance team complete the validation protocols.
What is the cavity count for 10ml bottles?
Depending on the bottle diameter, we typically run 4 to 8 cavities for 10ml bottles on the HGY50 platform, ensuring high output.
Can I use recycled material?
For pharma applications, virgin material is standard. However, the machine can process rPET if your regulatory body allows it for your specific application.
How do you ensure wall thickness uniformity?
The core rod ensures internal dimensions, while the mold defines the external. The “Stretch” phase in ISBM aligns the molecules, providing more uniform thickness than extrusion blowing.
14. Critical Companion: Class 0 Oil-Free Air Compressors
In pharmaceutical Injection Blow Molding Machine (One Step) operations, the compressed air touches the inside of every bottle. It must be sterile and absolutely oil-free.
We supply integrated Class 0 Oil-Free High-Pressure Air Compressors designed to meet ISO 8573-1 standards. Ensure your air quality matches your molding quality.
Secure Your Supply Chain with Ever-power Precision
Don’t compromise on sterility or precision. Partner with the experts in pharmaceutical ISBM technology.

